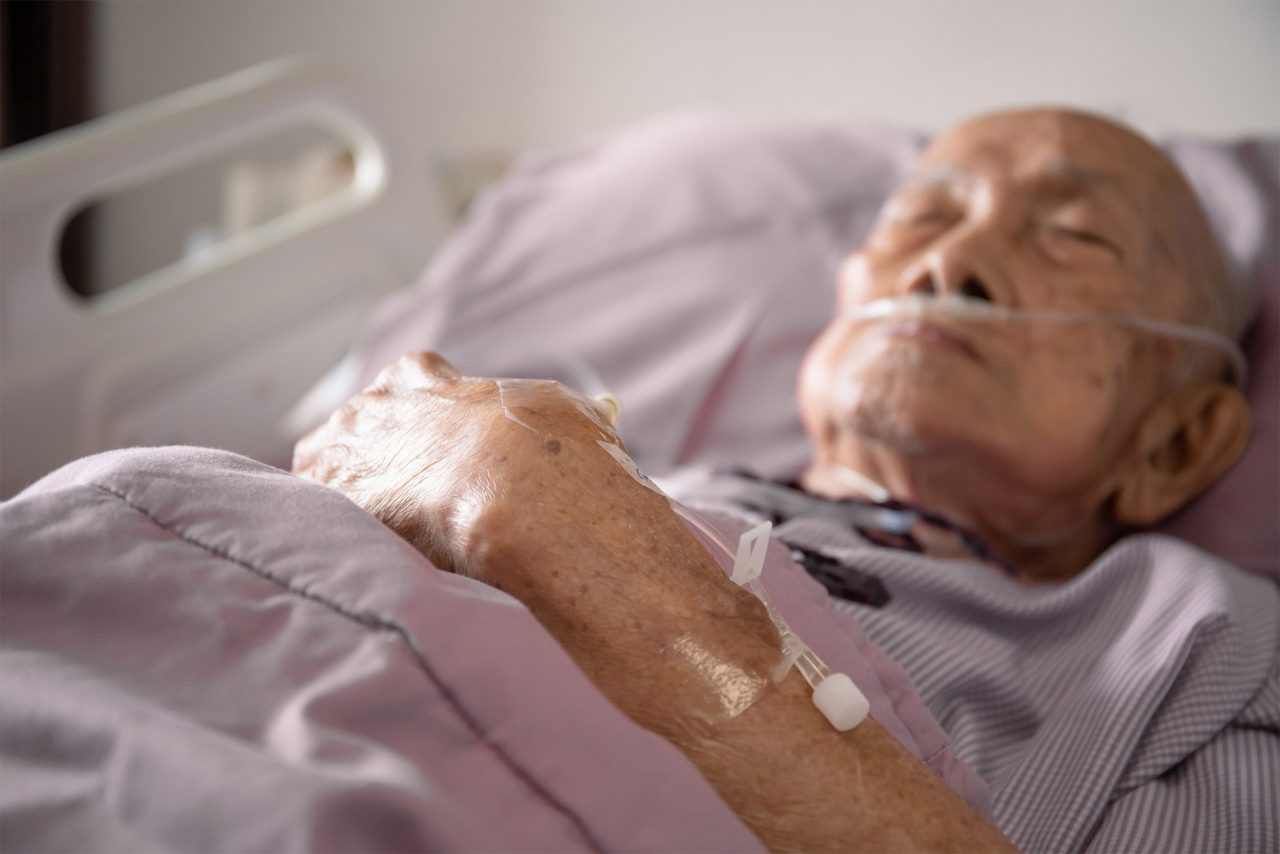
Researchers have found a way to predict if a patient will develop postoperative delirium, a common complication in older patients.
iStock by Getty Images
Health
Alzheimer’s disease biomarkers can predict post-op delirium
Research also provides new insights into how this delirium occurs
A clinical study led by investigators at Massachusetts General Hospital reveals that two newly identified plasma biomarkers of Alzheimer’s disease can predict postoperative delirium, one of the most common postoperative complications in older patients.
The findings are published in Annals of Surgery.
Clinical studies have already demonstrated a strong association between Alzheimer’s disease and delirium. A change to the Tau protein, which can lead to the formation of tangles in the brain, is one of the hallmarks of Alzheimer’s disease pathology, and Tau phosphorylation at threonine 217 (Tau-PT217) and threonine 181 (Tau-PT181) are new plasma biomarkers that can detect early-stage Alzheimer’s disease.
Previous studies from the same research group at MGH have shown that the ratio of beta amyloid (which causes Alzheimer’s disease signature plaques) to Tau in cerebrospinal fluid is associated with postoperative delirium. Additional studies in other labs have reported that plasma Tau-PT181 concentration distinguishes Alzheimer’s dementia from other neurological disorders. Plasma levels of Tau-PT217 are associated with the changes in cerebrospinal fluid levels of Tau-PT217 and Alzheimer’s development.
In this current study, the team at MGH use a novel method to measure Tau-PT217 and Tau-PT181 concentrations in plasma of patients. Called nanoneedle technology, it was developed in collaboration with Woburn-based NanoMosaic.
“Postoperative delirium could be a clinical manifestation of preclinical AD (Alzheimer’s disease) and might serve as a useful early warning sign to patients.”
Oluwaseun Akeju, co-author of the study
“The nanoneedle technology is ultrasensitive, requires a small volume, and can measure low concentrations of molecules, including Tau-PT217 and Tau-PT181,” says lead author Feng Liang from the Department of Anesthesia, Critical Care and Pain Medicine at MGH. “More than 20,000 nanoneedles are integrated on a silicon substrate assigned to detect one analyte. Each nanoneedle is a single molecule biosensor functionalized with antibodies,” Liang explained.
The group conducted experiments in 139 patients who had a knee replacement, hip replacement, or laminectomy (a type of back surgery) at MGH. They have discovered that preoperative plasma concentrations of Tau-PT217 and Tau-PT181 are associated with postoperative delirium. Moreover, Tau-PT217 is a stronger indicator of postoperative delirium than Tau-PT181.
“These outcomes help diagnosis of postoperative delirium; identify intermediate outcomes that could facilitate clinical studies; and elucidate the insight into potential mechanisms of postoperative delirium, ultimately leading to better and safer postoperative outcomes in patients,” said senior author Zhongcong Xie, the director of the Geriatric Anesthesia Research Unit in the MGH Department of Anesthesia, Critical Care and Pain Medicine.
“These data also suggest that Tau phosphorylation, contributes, at least partly, to the development of postoperative delirium,” said Xie, who is also the Henry K. Beecher Professor of Anesthesia at Harvard Medical School.
“Postoperative delirium could be a clinical manifestation of preclinical AD (Alzheimer’s disease) and might serve as a useful early warning sign to patients,” added co-author Oluwaseun Akeju, chair of the Department of Anesthesia, Critical Care and Pain Medicine.
High preoperative plasma concentrations of Tau-PT217 or Tau-PT181 can predict the presence and severity of postoperative delirium, with Tau-PT217 being the more strongly associated with these outcomes.
“We hope this work will promote more research to confirm these associations and to understand further the mechanisms underlying the interaction of delirium and AD, ultimately leading to better interventions for both,” says study co-author Edward R. Marcantonio from the Department of Medicine at Beth Israel Deaconess Medical Center. He is a professor of medicine at Harvard Medical School.
Additional co-authors are Kathryn Baldyga, Kathryn Cody, and Jeanine Wiener-Kronish from the MGH Department of Anesthesia, Critical Care and Pain Medicine; Qimin Quan from NanoMosaic, Yuan Shen from the MGH Department of Anesthesia, Critical Care and Pain Medicine and Anesthesia and Brain Research Institute, Shanghai Tenth People’s Hospital, Tongji University School of Medicine; Ashok Khatri and Shawn Choi from the MGH Endocrine Unit; and Brandon M. Westover from the MGH Department of Neurology.
The study was supported by the National Institutes of Health, including the National Institutes of Aging and the Eunice Kennedy Shriver National Institute of Child Health & Human Development.